News - 2020
2020, April the 14th
2020, April the 7th
Our last collaboration with IAME (Infection Antimicrobials Modelling Evolution, UMR1137) laboratory on the timing of antiviral treatment initiation to reduce SARS-Cov2 viral load has just been released in preprint.
We modeled the viral dynamics of 13 untreated patients infected with SARS-CoV-2 to infer viral growth parameters and predict the effects of antiviral treatments. In order to reduce peak viral load by more than 2 logs, drug efficacy needs to be greater than 80% if treatment is administered after symptom onset; an efficacy of 50% could be sufficient if treatment is initiated before symptom onset. Given their pharmacokinetic/pharmacodynamic properties, current investigated drugs may be in a range of 20-70% efficacy. They may help control virus if administered very early, but may not have a major effect in severe patients.
2020, April the 2nd
2020, March the 17th
Notre projet de Stratégie de repositionnement de médicaments pour le traitement des infections par le 2019-nCoV a été sélectionné parmi les 20 projets de recherche financés par REACTing pour lutter contre l’épidémie.
Avec le soutien du ministère des Solidarités et de la Santé et du ministère de l’Enseignement supérieur, de la Recherche et de l’Innovation, notre projet fait partie des 20 initiatives scientifiques qui ont été sélectionnées par le conseil scientifique de REACTing. Elles portent sur des thématiques aussi diverses que la modélisation de l’épidémie, la recherche de traitement ou la prévention.

2020, March the 9th
Development of new antiviral treatments against SARS-CoV2 in collaboration with the Center for Infection & Immunity of Lille (CIIL) and the Institut Pasteur de Lille.

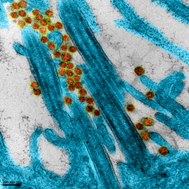
Jean Dubuisson's team at the Center for Infection and Immunity of Lille (CIIL) together with the Drug Discovery Center of Benoit Déprez on the campus of the Institut Pasteur de Lille and the VirPath team at the Centre International de Recherche en Infectiologie (CIRI) in Lyon join their complementary expertise and technologies for development of new antiviral treatments and fight more efficiently against SARS-CoV-2. VirPath has supplied the Center for Infection and Immunity of Lille with a characterized and sequenced SARS-CoV-2 strain and makes its preclinical models of infection available.
2020, March the 5th
VirPath on french TV news (France Television)
2020, March the 3rd
Signia Therapeutics highlighted in the Biotechfinances magazine n°889 about the Covid-19.
In the next days Signia Therapeutics is about to test two molecules of its pipeline against Coronavirus 2019-n-CoV , already validated in preclinical for their antiviral properties, especially against MERS-CoV and several other coronaviruses.
2020, March the 2nd
We were very honoured to receive Mr Alain Mérieux in our laboratory.
Nous avons été très honorés de recevoir Monsieur Alain Mérieux qui est venu encourager et féliciter toute l’équipe VirPath dans sa lutte contre le virus SARS-CoV-2. Ce moment d’échanges très convivial et bienveillant a été très apprécié des chercheurs et des étudiants. Nous remercions très sincèrement Monsieur Alain Mérieux, ainsi que le conseil scientifique de l’Institut Mérieux pour leur confiance dans nos travaux de recherche et leur soutien en nous accordant un « Mérieux Research grant ».
We were very honoured to receive Mr Alain Mérieux, who came to encourage and congratulate the VirPath team in its fight against the SARS-CoV-2. This very convivial and benevolent moment of exchange was greatly appreciated by both researchers and students. We sincerely thank Mr Alain Mérieux, as well as the scientific council of Institut Mérieux for their trust and support in our research work through the award of a “Mérieux Research grant”.




2020, February the 21st
The Lyon healthcare cluster is mobilizing alongside our #VirPath laboratory in its fight against SARS-CoV-2.
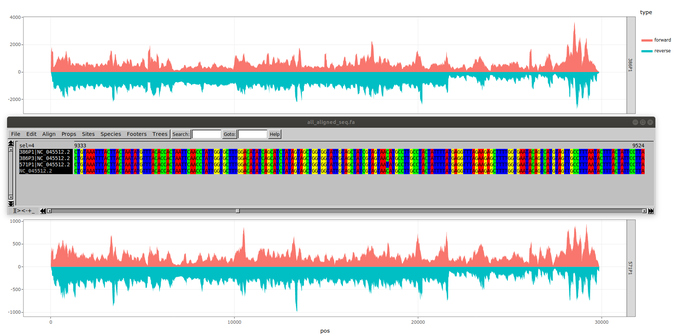
The Lyon healthcare cluster is mobilizing alongside our #VirPath laboratory in its fight against SARS-CoV-2. In collaboration with the Hospices Civils de Lyon, the genomic platform ProfileXpert and the companies ViroScan3D and Signia Therapeutics, our laboratory has isolated and characterized by deep sequencing two SARS-CoV-2 viral strains from several patient samples. Our analyses confirm that our SARS-CoV2 viral bank is genetically stable after amplification at both the consensus level and the low-frequency mutation landscape. Sequences have been uploaded to GISAID to contribute to the international program monitoring and studying the SARS-CoV-2.
2020, February the 19th
The BIOTECHFINANCES magazine highlighted the work of Signia Therapeutics and VirPath to fight against the coronavirus "2019-nCoV".
2020, February the 12th
Glycoflu program aims to develop a new generation of influenza antiviral drugs.
Our VirPath Laboratory, with its technological research Platform VirNext, is partner of the GlycoFlu program which aims to develop a new generation of influenza drugs that could be administered orally (aerosols) with multiple development potentials (human or avian flu) and applications both prophylactic and therapeutic. Launched on January 20, 2020, this program funded by the SATT Linksium Grenoble Alpes, is led by Emeline Richard Millot, winner of the Grand Prize in the I-PhD competition from the Ministry of Higher Education, Research and Innovation in partnership with BPI France, and Eric Samain from CERMAV (CNRS). #influenza #antiviral

2020, February the 10th
The Agence France-Presse (AFP) dispatch about VirPath is quoted by french and international newspapers.
2020, February the 6th
Video reportages of Agence France-Presse (AFP).
"VirPath, a university laboratory in Lyon, is working to find an effective treatment for the novel coronavirus using an existing database of drugs."
2020, February the 4th
Our laboratory VIRPATH is mobilized with the REACTING consortium on the front line against the 2019-n-CoV Coronavirus
We are currently characterizing the virus in several preclinical models of interest with the aim of applying our drug repurposing strategy and thus rapidly proposing a therapeutic solution to be tested in clinic against such new emerging epidemic virus. Signia Therapeutics joins our laboratory in the fight against Coronavirus 2019-n-CoV and announces that two proprietary molecules from its portfolio, already validated for their antiviral properties against MERS-COV and several other human and animal coronaviruses, will be tested against Coronavirus 2019-n-CoV in preclinical models of infection.
2020, February the 3rd
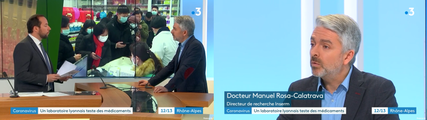
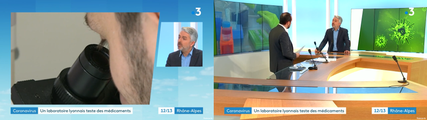
In Lyon, the VirPath laboratory tries to find a treatment against 2019-nCoV.
Dr Manuel ROSA-CALATRAVA has been interviewed by France 3 Auvergne Rhône Alpes about the novel coronavirus. He explained that the VirPath laboratory was focused on finding a treatment for coronavirus using existing drugs.

2020, January the 20th
INSERM press room : "Deadly outbreak in China linked to a novel form of coronavirus"
2020, January the 15th
Launch of Cynbiome network
VirPath: We are pleased to announce the launch of Cynbiome, a preclinical excellence network on microbiome and infectious diseases. We congratulate the principal investigator Cynbiose, a service company specializing in the development and commercialization of innovative preclinical models.
Our laboratory and our Technological Research Platform VirNext are very proud to be partners of the cynbiome network. We will bring our expertises, OMICS analytic tools and biological materials in the field of infectious diseases to contribute in the development of this ambitious and unique network in EU.
2020, January the 15th
Our last editorial just published in John Libbey Eurotext Virologie concerning Novel antiviral compounds and combination therapy for influenza viruses.
Notre éditorial dans Virologie, la revue officielle de la Société Française de Virologie, sur les Nouveaux antiviraux et thérapie combinée contre les virus influenza.